Abstract
Background: The natural history of low grade non-Hodgkin lymphoma (NHL) typically includes multiple relapses and short responses to therapy after each relapse. The development of novel therapies for NHL patients with multiple relapses is therefore clinically needed. In previous studies, we demonstrated the presence of PD-1 positive exhausted T cells in the nodal tissue of low grade NHL patients. We therefore hypothesized that blocking PD-1 will activate tumor infiltrating T cells to induce effector cell-mediated cytotoxicity. To test this hypothesis, we performed a phase 2 trial (MC1485) to test the efficacy of the PD-1 blocking antibody pembrolizumab in relapsed/refractory low grade NHL.
Methods: Relapsed/refractory low grade NHL including follicular lymphoma (FL), marginal zone lymphoma (MZL) and lymphoplasmacytic lymphoma (LPL) who had ECOG performance 0-2 and normal organ function were included in arm B of the MC1485 trial. Patients with prior allogeneic stem cell transplant were excluded. Pembrolizumab 200 mg was administered intravenously every 3 weeks until progression, excessive toxicity, or completion of 2 years of therapy. The primary end point of this study was overall response rate. Tumor T cell infiltration and expression of PD-1 and PD-L1 were assessed with standard immunohistochemical (IHC) staining.
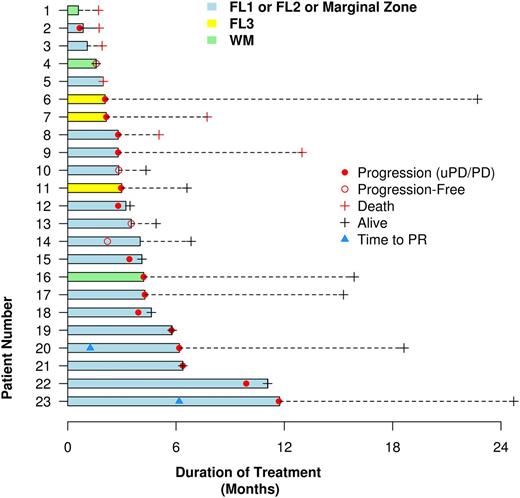
Results: 23 patients including 18 FL, 2 MZL and 3 LPL were enrolled and the median age was 67 years (47-82). The median number of prior therapies was 4 (1-20). 17 out of 23 (74%) patients had received more than 2 prior therapies. 100% of patients had received an anti-CD20 antibody and 22/23 (96%) had received chemoimmunotherapy. 6 (26%) had a prior autologous stem cell transplant. 10 (43%) had received prior novel agents including lenalidomide or idelalisib or other experimental therapies. The median number of pembrolizumab doses received was 4 (range, 1 to 16), administered over a median treatment duration of 13.9 weeks (range, 2.6 to 50.8). Drug-related adverse events (AE) occurred in 17 patients (74%) with the common ones being thrombocytopenia in 6 (26%), neutropenia in 5 (22%), anemia in 5 (22%), nausea in 5 (22%), diarrhea in 4 (17%), lymphocyte decrease in 4 (17%), nausea, vomiting and cough each in 3 (13%), and dyspnea in 2 (8%) patients. Drug-related grade 3 or above AE occurred in 9 (39%) patients with most common ones being thrombocytopenia (3, 13%), anemia (3, 13%), neutropenia (2, 8%) and dyspnea (2, 8%). One possibly related death occurred in a patient who had baseline obstructive sleep apnea and developed respiratory failure after two doses of therapy without clear cause. He eventually refused to receive further supportive care. Based on the investigator assessment, 2 FL patients had partial responses (PR, 9%). One LPL patient had minor response using LPL/Waldenstrom macroglobulinemia response criteria (MR, 4%). 11 patients (48%) had stable disease (SD). These two responders had durations of response of 5.5 and 4.9 months respectively (Figure). One MZL patient had ~40% tumor reduction with ongoing therapy. 7 patients (30%) had progressive disease. With a median follow-up being 6.5 months (1.6-24.7), the median progression free survival is 3.4 months (95% CI: 2.1-5.7). The median overall survival has not been reached.
Conclusion: Pembrolizumab had an acceptable safety profile in low grade NHL patients. In selected NHL patients, pembrolizumab has activity as single-agent therapy. Biomarker evaluation is needed to select patients who can benefit from this therapy. Further combination therapy will be required to improve efficacy.
Ding: Merck: Research Funding. Witzig: Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Research Funding; Kura: Research Funding; Novartis: Research Funding. Nowakowski: Bayer: Consultancy, Research Funding; Abbvie: Consultancy; celgene: Consultancy, Research Funding; Morphosys: Consultancy, Research Funding; Genetech: Consultancy, Research Funding; pharmacyclics: Consultancy; Nanostring: Research Funding. Reeder: Millennium: Research Funding; Celgene: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Novartis: Research Funding. Kapoor: Takeda, Celgene and Amgen: Research Funding. Ansell: Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Merck: Research Funding; Celldex: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal